IB Chemistry SL - 2024 - Questionbank
Topic 2 All - Atomic Structure
All Questions for Topic 2 (Atomic Structure). The Nuclear Atom, Electron Configuration
Question Type
All
Paper
Difficulty
View
Question 1
What is the maximum number of electrons in the subshell?
-
A. 6
-
B. 10
-
C. 2
-
D. 8
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 2
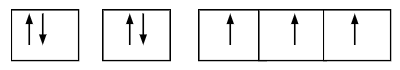
What is the electron configuration of this orbital diagram?
-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 3
Which of the following subshells contains the highest energy?
-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 4
The atom of an element Q has an atomic number of 27 and a mass number of 60. Which of the following statements is true about the subatomic particles of the atom of element Q?
-
I. Atom of element Q has 27 electrons and 60 protons II. Atom of element Q has 27 protons and 27 electrons III. Atom of element Q has 27 electrons and 60 neutrons IV. Atom of element Q has 27 protons and 33 neutrons
-
A. I and IV only
-
B.II and IV only
-
C.II and III only
-
D.I and III only
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 5
Which of the following is most likely to be the diameter of a carbon nucleus, if the diameter of a particular carbon atom is measured as 140 pm?
-
A. 12 amu
-
B. 0.0012 mm
-
C. 0.001 nm
-
D. 0.005 pm
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 6
In Rutherford’s gold foil experiment, some alpha particles bounced back towards the source. Which of the following statements best explains this result?
-
A. The atom consists mainly of empty space.
-
B. The atom has a positively charged center.
-
C. The atom consists of negative particles around the center.
-
D. The atom has a neutrally charged core.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 7
Which of the following statements is not true?
-
I. Electrons have greater mass than protons and neutrons II. Protons and neutrons are in the nucleus III. Protons and electrons are charged subatomic particles IV. Electrons are located outside the nucleus
-
A.I and III only
-
B.II and IV only
-
C.II only
-
D.I only
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 8
What is the term for the region where there is the highest probability of finding an electron?
-
A. Orbital
-
B. Nucleus
-
C. Orbital diagram
-
D. Orbit
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 9
Which of the following is the correct electron configuration of a typical sodium ion?
-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 10
The nuclear symbol for an isotope of iodine is . Which of the following is correct?
| Atomic Number | Mass Number | Number of protons | Number of neutrons | Number of electrons | |
|---|---|---|---|---|---|
| A. | 53 | 125 | 53 | 72 | 53 |
| B. | 53 | 125 | 72 | 53 | 53 |
| C. | 125 | 53 | 53 | 72 | 72 |
| D. | 125 | 53 | 53 | 53 | 72 |
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 11
A mass spectrometer was used to determine that the relative ratio by mass of N:O in a molecule containing nitrogen and oxygen is 7:16. What is the most likely molecule?
-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 12
Which of the following is the correct orbital diagram for ?
-
A. 
B. 
C. 
D. 
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 13
Which of the following is not a physical property of an isotope of an element with fewer neutrons in its atom?
-
A. Lower mass
-
B. Faster rate of diffusion
-
C. Higher density
-
D. Lower melting and boiling points
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 14
[Maximum mark: 2]
Cobalt is an important cofactor that often inhibits enzyme activity involved in cellular respiration, such as catalase.
-
Using the complete electron configuration, determine the electron configuration of [1]
-
Draw the orbital diagram of the electrons of . [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 15
The isotopes of carbon and :
-
I. have the same atomic number II. have the same physical properties III. have the same chemical properties
-
A.I and III only
-
B.I and II only
-
C.II and III only
-
D.I, II and III
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 16
How many electrons are there in ?
-
A. 17
-
B. 18
-
C. 35
-
D. 36
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 17
The following table shows the number of subatomic particles present in five species.
| Species | Number of protons | Number of neutrons | Number of electrons |
|---|---|---|---|
| A | 8 | 8 | 8 |
| B | 8 | 8 | 10 |
| C | 9 | 10 | 8 |
| D | 10 | 10 | 10 |
| E | 10 | 12 | 10 |
Which two species are isotopes of the same element?
-
A.A and B
-
B.B and C
-
C.C and D
-
D.D and E
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 18
[Maximum mark: 4]
Hydrogen exhibits two emission lines, and . has a wavelength of and has a frequency of .
-
Calculate the frequency of the emission line of hydrogen. [1]
-
Calculate the wavelength of the emission line of hydrogen. [1]
-
Compare the two emission lines, and using the Data Booklet, suggest which of the emission lines is most likely to be red. [2]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 19
The following table shows the number of subatomic particles present in five species.
| Species | Number of protons | Number of neutrons | Number of electrons |
|---|---|---|---|
| A | 8 | 8 | 8 |
| B | 8 | 8 | 10 |
| C | 9 | 10 | 8 |
| D | 10 | 10 | 10 |
| E | 10 | 12 | 10 |
Which species are ions?
-
A.A and B
-
B.B and C
-
C.C and D
-
D.D and E
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 20
What will happen to the frequency of moving light particles if the wavelength is doubled, assuming the velocity is constant?
-
A. The frequency will be doubled
-
B. The frequency will decrease by half
-
C. The frequency will not be affected
-
D. The frequency will increase by four
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 21
Which of the following statement(s) is (are) true?
- I. The emission spectra are produced from a release of energy of excited electrons
II. No two electrons in one atom have the same set of quantum numbers
III. The electrons in an orbital must have an opposite spin
-
A. I only
-
B. II and III only
-
C. I and II only
-
D. I, II, and III
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 22
Mass spectrometry uses fast-moving electrons to ionize particles and determine the mass-to-charge ratio of an unknown compound.
-
Using the data booklet section 1 and 2, calculate the frequency of an electron with a wavelength of [1]
-
Calculate the wavelength of an electron with a frequency of . [1]
-
State the effect of increasing the wavelength on frequency. [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 23
Which of the following statements is incorrect?
-
A.All ions contain electrons
-
B.A few alpha particles deflected at large angles in Rutherford's Gold Foil experiment
-
C.A mass spectrometer can tell us the number of isotopes an element has
-
D.An atom can have more neutrons than protons
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 24
[Maximum mark: 5]
The atom of element X has a mass number of 127 and has 74 neutrons. The ion derived from X has 54 electrons.
-
Calculate the number of protons of element X. [1]
-
State the nuclear symbol of the ion formed (refer to the periodic table). [2]
-
An isotope of X has a mass number of 132. Determine the number of neutrons in its atom. [2]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 25
The following is a hydrogen energy level diagram.

How many different emission lines are possible?
-
A.1
-
B.5
-
C.15
-
D.30
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 26
[Maximum mark: 8]
Deuterium is an isotope of hydrogen that contains one proton, one neutron, and one electron.
-
-
Complete the following table. [2]
Protons Neutrons Electrons Relative charge Relative mass ~ Location -
Using the following data, calculate the relative atomic mass of hydrogen correct to five decimal places, showing your work. [2]
Isotope Relative abundance Hydrogen-1 99.98% Deuterium 0.012% Hydrogen-3 0.008%
-
-
-
Describe the emission spectrum of the hydrogen atom. [2]
-
Outline the difference between a continuous spectrum and a line spectrum. [1]
-
Suggest how your answer to (b)(i) shows that energy levels are quantized. [1]
-
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 27
[Maximum mark: 9]
Cobalt-60 is a radioactive isotope of the element cobalt that is used to treat thyroid cancer.
-
-
State the full electron configuration of cobalt and chromium. [2]
-
Explain what is special about the electron configuration of chromium. [1]
-
State the atomic symbol notation for the cobalt-60 isotope. [1]
-
One atom of cobalt-60 undergoes beta decay, releasing an electron and energy with a frequency of to form nickel-60.
-
-
Using section 1 of the data booklet, calculate the wavelength of energy released from beta decay. [1]
-
Using section 3 (Section 5- 2025 Syllabus) of the data booklet and your answer to (b) (i), deduce the type of electromagnetic radiation emitted. [1]
-
-
-
Define orbital. [1]
-
Sketch the shapes of an s-orbital and a p-orbital. [2]
-
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 28
[Maximum mark: 5]
Each element contains a different arrangement of electrons, and these electron configurations can also be represented using orbital diagrams. Determine the identity of each element described below.
-
[1]
-
[1]
-
In Period with valence electrons of [1]
-
 [1]
[1]
-
An ion with +1 charge, with valence electrons of:
 [1]
[1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Video (d)
Video (e)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 29
[Maximum mark: 5]
Complete the following table:
| Nuclear Symbol | Number of protons | Number of neutrons | Number of electrons |
|---|---|---|---|
| 62 | |||
| 81 | 120 | ||
| 26 | |||
| 46 | |||
| 16 | 16 | 18 |
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 30
Which of the following pairs of terms describe the shape of an s orbital and the relative energy of a d orbital?
| A. | Spherical | Low |
| B. | Dumbbell-shape | High |
| C. | Spherical | High |
| D. | Dumbbell-shape | Low |
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 31
Which atoms' orbital diagram does this figure represent?

-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Video
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 32
[Maximum mark: 7]
and are the two most abundant isotopes of copper.
-
-
Define isotope. [1]
-
Determine the correct numbers of subatomic particles to complete the following table. [1]
Copper-63 Copper-65 Number of protons Number of electrons Number of neutrons -
Calculate the relative abundances of and using section 6 (Section 7- 2025 Syllabus) of the data booklet. [3]
-
-
Complete the orbital diagram for the valence electrons in a copper atom and a copper (II) ion. [2]

Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 33
[Maximum mark: 3]
is a transition metal commonly used in wires.
-
Determine the complete electron configuration of . [1]
-
State the condensed electron configuration of [1]
-
Determine whether the electron configuration of follows the usual rules of electron configuration. [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 34
[Maximum mark: 4]
John is sitting on a beach on a warm summer evening watching the reddish sky () while his brother, Eric, is playing with his laser beam with a frequency of .
-
Calculate the frequency of the radiation from the red sky. [1]
-
Calculate the wavelength of the laser beam of Eric. [1]
-
Compare the wavelength of the laser beam with the wavelength of the radiation of the red sky. [1]
-
Deduce the colour of the laser beam of Eric. [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Video (d)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 35
What is the smallest amount of energy an electron can release?

-
A. 0.31
-
B. 0.54
-
C. 10.19
-
D. 13.59
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 36
[Maximum mark: 5]
Lithium is a low-density alkali metal that reacts vigorously with water.
- Given the below data and referring to the periodic table, calculate the relative abundances of the isotopes of lithium. [3]
| Isotope | Atomic Mass |
|---|---|
| 6.015 | |
| 7.016 |
-
State the definition of an isotope. [1]
-
Outline the concept of relative abundance. [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 37
[Maximum mark: 5]
Element Y has 4 stable isotopes. The table below shows the relative abundance and atomic masses of these isotopes.
| Isotope | Percent abundance | Atomic mass |
|---|---|---|
| 1 | 1.40 % | 203.97 |
| 2 | 24.10 % | 205.97 |
| 3 | 22.10 % | 206.98 |
| 4 | 52.40 % | 207.98 |
-
Calculate the relative atomic mass of the element. [2]
-
Identify the element. [1]
-
The atom of the element can form an ion with a charge of 4+. Determine the number of electrons in the ion. [1]
-
Determine the nuclear symbol of another corresponding ion of element Y given the numbers of subatomic particles: electrons = 80; neutrons = 122. [1]
Formula Booklet
Mark Scheme
Video (a)
Video (b)
Video (c)
Video (d)
Video (d)
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Solutions
Revisit
Ask Newton
Question 38
[Maximum mark: 10]
Copper(II) chloride can exist as an anhydrous solid or hydrate.
-
A sample of copper(II) chloride hydrate was heated on a watch glass until the blue solid changed completely to brown, and the following data was recorded:
Mass of watch glass () Mass of copper(II) chloride hydrate () Mass of anhydrous copper(II) chloride + watch glass (1st heating, 5 min) Mass of anhydrous copper(II) chloride + watch glass (2nd heating, 5 min) Mass of anhydrous copper(II) chloride + watch glass (3rd heating, 2 min) Suggest a reason why the sample was heated for a second 5 minute increment showing a mass change. [1]
-
Calculate the mass of anhydrous copper(II) chloride. Do not include the calculations of uncertainties in your work. [1]
-
Calculate the mass of water in the hydrated copper(II) chloride sample. Do not include the calculations of uncertainties in your work. [1]
-
Calculate the percent uncertainty on the mass of water in the copper(II) chloride sample. [2]
-
Determine the chemical formula for the copper(II) chloride hydrate from the experimental data. [3]
-
State the chemical name of the copper(II) chloride hydrate from this experiment. [1]
-
State the core electron configuration for copper. [1]
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 39
What is the relative atomic mass, , of an unknown element found in a meteorite with the isotopic abundance shown below?
| Isoptope | Abundance% |
|---|---|
| 80.0 | |
| 1.00 | |
| 19.0 |
-
A. 39.00
-
B. 40.00
-
C. 39.39
-
D. 40.50
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Question 40
Which electron transition in the hydrogen atom emission spectrum emits radiation with the highest frequency?
-
A.
-
B.
-
C.
-
D.
Formula Booklet
Mark Scheme
Revisit
Check with RV Newton
Formula Booklet
Mark Scheme
Revisit
Ask Newton
Thank you Revision Village Members
#1 IB Math Resource
Revision Village is ranked the #1 IB Math Resources by IB Students & Teachers.
34% Grade Increase
Revision Village students scored 34% greater than the IB Global Average in their exams (2021).
80% of IB Students
More and more IB students are using Revision Village to prepare for their IB Math Exams.
More IB Chemistry SL - 2024 Resources
Frequently Asked Questions
What is the IB Chemistry SL Questionbank?
The IB Chemistry SL Questionbank is a comprehensive set of IB Chemistry exam style questions, categorised into syllabus topic and concept, and sorted by difficulty of question. The bank of exam style questions are accompanied by high quality step-by-step markschemes and video tutorials, taught by experienced IB Chemistry teachers. The IB Chemistry SL Question bank is the perfect exam revision resource for IB students looking to practice IB Chemistry exam style questions in a particular topic or concept in their IB Chemistry Standard Level course.
Where should I start in the Chemistry SL Questionbank?
The IB Chemistry SL Questionbank is designed to help IB students practice Chemistry SL exam style questions in a specific topic or concept. Therefore, a good place to start is by identifying a concept that you would like to practice and improve in and go to that area of the Chemistry SL Question bank. For example, if you want to practice Chemistry SL exam style questions covering Electron Configuration, you can go to Chemistry SL Topic 2 (Atomic Structure) and go to the Electron Configuration area of the question bank. On this page there is a carefully designed set of IB Chemistry SL exam style questions, progressing in order of difficulty from easiest to hardest. If you’re just getting started with your revision, you could start at the top of the page with the easiest questions, or if you already have some confidence, you could start at the medium difficulty questions and progress down.
How should I use the Chemistry SL Questionbank?
The Chemistry SL Questionbank is perfect for revising a particular topic or concept, in-depth. For example, if you wanted to improve your knowledge of Electron Configuration, there are over 20 IB Chemistry SL exam style questions focused specifically on this concept. Alternatively, Revision Village also has an extensive library of Chemistry SL Practice Exams, where students can simulate the length and difficulty of an IB exam with the Mock Exam sets, as well as Chemistry SL Key Concepts, where students can learn and revise the underlying theory, if missed or misunderstood in class.
What if I finish the Chemistry SL Questionbank?
With an extensive and growing library of full length IB Chemistry SL exam style questions in the Chemistry SL Question bank, finishing all of the questions would be a fantastic effort, and you will be in a great position for your final exams. If you were able to complete all the questions in the Chemistry SL Question bank, then a popular option would be to go to the Chemistry SL Practice Exams section on Revision Village and test yourself with the Mock Exam Papers, to simulate the length and difficulty of an actual IB Chemistry SL exam.